Ideal gas 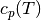 polynomials for various fluids¶
polynomials for various fluids¶
For the polynomial
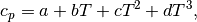
the values of 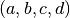 are tabulated below. Note that
are tabulated below. Note that 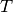 is in K, and
is in K, and 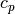 is in J/mol/K. This table is originally located at
http://www.wiley.com/college/moran/CL_0471465704_S/user/tables/TABLE3S/table3sframe.html.
It has been reproduced here for the sake of posterity.
is in J/mol/K. This table is originally located at
http://www.wiley.com/college/moran/CL_0471465704_S/user/tables/TABLE3S/table3sframe.html.
It has been reproduced here for the sake of posterity.
| Substance | a | b | c | d | e | Error (%) | ||
|---|---|---|---|---|---|---|---|---|
| Range (K) | Max. | Avg. | ||||||
| Nitrogen | N2 | 28.9 | -0.1571 X 10-2 | 0.8081 X 10-5 | -2.873 X 10-9 | 273-1800 | 0.59 | 0.34 |
| Oxygen | O2 | 25.48 | 1.520 X 10-2 | -0.7155 X 10-5 | 1.312 X 10-9 | 273-1800 | 1.19 | 0.28 |
| Air | 28.11 | 0.1967 X 10-2 | 0.4802 X 10-5 | -1.966 X 10-9 | 273-1800 | 0.72 | 0.33 | |
| Hydrogen | H2 | 29.11 | -0.1916 X 10-2 | 0.4003 X 10-5 | -0.8704 X 10-9 | 273-1800 | 1.01 | 0.26 |
| Carbon monoxide | CO | 28.16 | 0.1675 X 10-2 | 0.5372 X 10-5 | 2.222 X 10-9 | 273-1800 | 0.89 | 0.37 |
| Carbon dioxide | CO2 | 22.26 | 5.981 X 10-2 | -3.501 X 10-5 | 7.469 X 10-9 | 273-1800 | 0.67 | 0.22 |
| Water vapor | H2O | 32.24 | 0.1923 X 10-2 | 1.055 X 10-5 | -3.595 X 10-9 | 273-1800 | 0.53 | 0.24 |
| Nitric oxide | NO | 29.34 | -0.09395 X 10-2 | 0.9747 X 10-5 | -4.187 X 10-9 | 273-1500 | 0.97 | 0.36 |
| Nitrous oxide | N2O | 24.11 | 5.8632 X 10-2 | -3.562 X 10-5 | 10.58 X 10-9 | 273-1500 | 0.59 | 0.26 |
| Nitrogen dioxide | NO2 | 22.9 | 5.715 X 10-2 | -3.52 X 10-5 | 7.87 X 10-9 | 273-1500 | 0.46 | 0.18 |
| Ammonia | NH3 | 27.568 | 2.5630 X 10-2 | 0.99072 X 10-5 | -6.6909 X 10-9 | 273-1500 | 0.91 | 0.36 |
| Sulfur | S2 | 27.21 | 2.218 X 10-2 | -1.628 X 10-5 | 3.986 X 10-9 | 273-1800 | 0.99 | 0.38 |
| Sulfur dioxide | SO2 | 25.78 | 5.795 X 10-2 | -3.812 X 10-5 | 8.612 X 10-9 | 273-1800 | 0.45 | 0.24 |
| Sulfur trioxide | SO3 | 16.4 | 14.58 X 10-2 | -11.20 X 10-5 | 32.42 X 10-9 | 273-1300 | 0.29 | 0.13 |
| Acetylene | C2H2 | 21.8 | 9.2143 X 10-2 | -6.527 X 10-5 | 18.21 X 10-9 | 273-1500 | 1.46 | 0.59 |
| Benzene | C6H6 | -36.22 | 48.475 X 10-2 | -31.57 X 10-5 | 77.62 X 10-9 | 273-1500 | 0.34 | 0.2 |
| Methanol | CH4O | 19 | 9.152 X 10-2 | -1.22 X 10-5 | -8.039 X 10-9 | 273-1000 | 0.18 | 0.08 |
| Ethanol | C2H6O | 19.9 | 20.96 X 10-2 | -10.38 X 10-5 | 20.05 X 10-9 | 273-1500 | 0.4 | 0.22 |
| Hydrogen chloride | HCl | 30.33 | -0.7620 X 10-2 | 1.327 X 10-5 | -4.338 X 10-9 | 273-1500 | 0.22 | 0.08 |
| Methane | CH4 | 19.89 | 5.024 X 10-2 | 1.269 X 10-5 | -11.01 X 10-9 | 273-1500 | 1.33 | 0.57 |
| Ethane | C2H6 | 6.9 | 17.27 X 10-2 | -6.406 X 10-5 | 7.285 X 10-9 | 273-1500 | 0.83 | 0.28 |
| Propane | C3H8 | -4.04 | 30.48 X 10-2 | -15.72 X 10-5 | 31.74 X 10-9 | 273-1500 | 0.4 | 0.12 |
| n-Butane | C4H10 | 3.96 | 37.15 X 10-2 | -18.34 X 10-5 | 35.00 X 10-9 | 273-1500 | 0.54 | 0.24 |
| i-Butane | C4H10 | -7.913 | 41.60 X 10-2 | -23.01 X 10-5 | 49.91 X 10-9 | 273-1500 | 0.25 | 0.13 |
| n-Pentane | C5H12 | 6.774 | 45.43 X 10-2 | -22.46 X 10-3 | 42.29 X 10-9 | 273-1500 | 0.56 | 0.21 |
| n-Hexane | C6H14 | 6.938 | 55.22 X 10-2 | -28.65 X 10-5 | 57.69 X 10-9 | 273-1500 | 0.72 | 0.2 |
| Ethylene | C2H4 | 3.95 | 15.64 X 10-2 | -8.344 X 10-5 | 17.67 X 10-9 | 273-1500 | 0.54 | 0.13 |
| Propylene | C3H6 | 3.15 | 23.83 X 10-2 | -12.18 X 10-5 | 24.62 X 10-9 | 273-1500 | 0.73 | 0.17 |